EQUILIBRIUM IN CHEMICAL PROCESSES – DYNAMIC EQUILIBRIUM
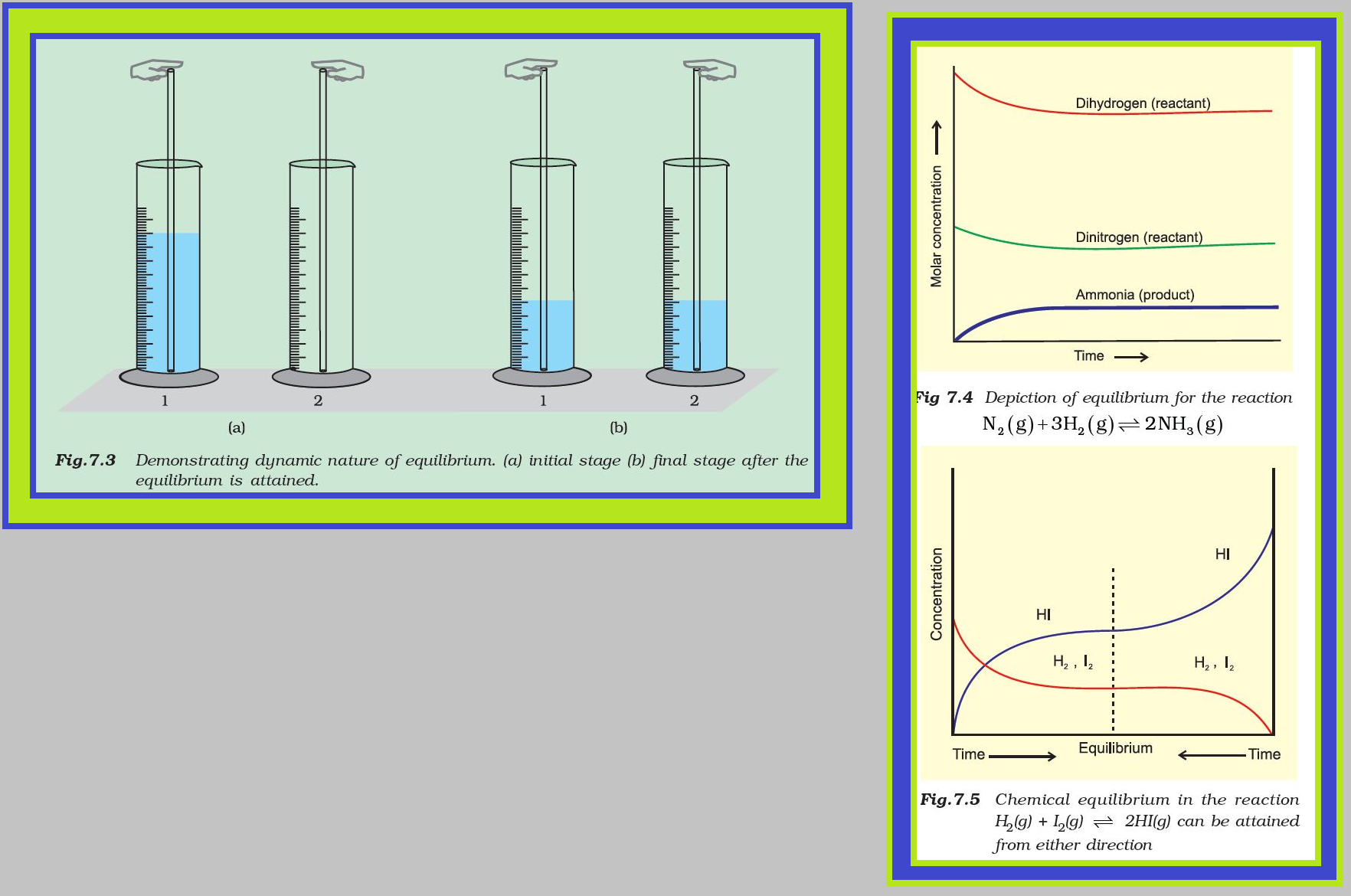
The chemical reactions can occur both in forward and backward directions. When the rates of the forward and reverse reactions become equal, the concentrations of the reactants and the products remain constant. This is the stage of chemical equilibrium( dynamic equilibrium).
For a better comprehension, let us consider a general case of a reversible reaction,
`color{red}(A + B ⇌ C + D)`
With passage of time, there is accumulation of the products C and D and depletion of the reactants A and B (Fig. 7.2). This leads to a decrease in the rate of forward reaction and an increase in he rate of the reverse reaction

Eventually, the two reactions occur at the same rate and the system reaches a state of equilibrium.
Similarly, the reaction can reach the state of equilibrium even if we start with only C and D; that is, no A and B being present initially, as the equilibrium can be reached from either direction.
The dynamic nature of chemical equilibrium can be demonstrated in the synthesis of ammonia by Haber’s process. In a series of experiments, Haber started with known amounts of dinitrogen and dihydrogen maintained at high temperature and pressure and at regular intervals determined the amount of ammonia present. He was successful in determining also the concentration of unreacted dihydrogen and dinitrogen. Fig. 7.4 shows that after a certain time the composition of the mixture remains the same even though some of the reactants are still present. This constancy in composition indicates that the reaction has reached equilibrium. In order to understand the dynamic nature of the reaction, synthesis of ammonia is carried out with exactly the same starting conditions (of partial pressure and temperature) but using `color{red}(D_2)` (deuterium) in place of `color{red}(H_2)`. The reaction mixtures starting either with `color{red}(H_2)` or `color{red}(D_2)` reach equilibrium with the same composition, except that `color{red}(D_2)` and `color{red}(ND_3)` are present instead of `color{red}(H_2)` and `color{red}(NH_3)`. After equilibrium is attained, these two mixtures (`color{red}(H_2, N_2, NH_3)` and `color{red}(D_2, N_2, ND_3)`) are mixed together and left for a while. Later, when this mixture is analysed, it is found that the concentration of ammonia is just the same as before. However, when this mixture is analysed by a mass spectrometer, it is found that ammonia and all deuterium containing forms of ammonia (`color{red}(NH_3, NH_2D, NHD_2)` and `color{red}(ND_3)`) and dihydrogen and its deutrated forms (`color{red}(H_2, HD)` and `color{red}(D_2)`) are present. Thus one can conclude that scrambling of `color{red}(H)` and `color{red}(D)` atoms in the molecules must result from a continuation of the forward and reverse reactions in the mixture. If the reaction had simply stopped when they reached equilibrium, then there would have been no mixing of isotopes in this way.
Use of isotope (deuterium) in the formation of ammonia clearly indicates that chemical reactions reach a state of dynamic equilibrium in which the rates of forward and reverse reactions are equal and there is no net change in composition.
Equilibrium can be attained from both sides, whether we start reaction by taking, `color{red}(H_2(g))` and `color{red}(N_2(g))` and get `color{red}(NH_3(g))` or by taking
`color{red}(NH_3(g))` and decomposing it into `color{red}(N_2(g))` and `color{red}(H_2(g))`.
`color{red}(N_2 (g) +3H_2 (g) ⇌ 2NH_3 (g))`
`color{red}(2NH_3 (g) ⇌ N_2 (g) +3H_2 (g)) `
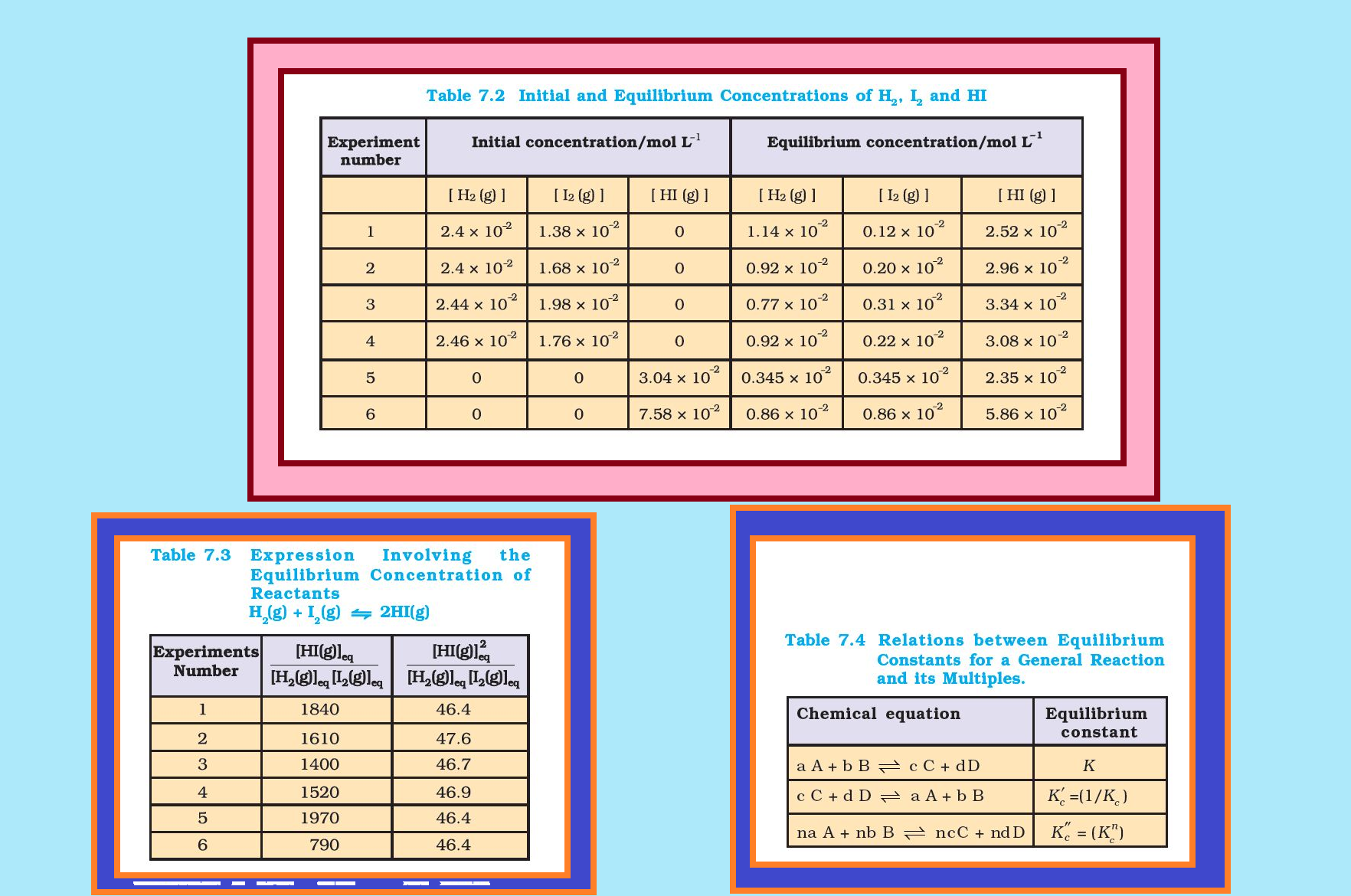
Similarly let us consider the reaction, `color{red}(H_2(g) + I_2(g) ⇌ 2HI(g))`. If we start with equal initial concentration of `color{red}(H_2)` and `color{red}(I_2)`, the reaction proceeds in the forward direction and the concentration of `color{red}(H_2)` and `color{red}(I_2)` decreases while that of `color{red}(HI)` increases, until all of these become constant at equilibrium (Fig. 7.5). We can also start with `color{red}(HI)` alone and make the reaction to proceed in the reverse direction; the concentration of `color{red}(HI)` will decrease and concentration of `color{red}(H_2)` and `color{red}(I_2)` will increase until they all become constant when equilibrium is reached (Fig.7.5). If total number of `color{red}(H)` and `color{red}(I)` atoms are same in a given volume, the same equilibrium mixture is obtained whether we start it from pure reactants or pure product.
For a better comprehension, let us consider a general case of a reversible reaction,
`color{red}(A + B ⇌ C + D)`
With passage of time, there is accumulation of the products C and D and depletion of the reactants A and B (Fig. 7.2). This leads to a decrease in the rate of forward reaction and an increase in he rate of the reverse reaction

Eventually, the two reactions occur at the same rate and the system reaches a state of equilibrium.
Similarly, the reaction can reach the state of equilibrium even if we start with only C and D; that is, no A and B being present initially, as the equilibrium can be reached from either direction.
The dynamic nature of chemical equilibrium can be demonstrated in the synthesis of ammonia by Haber’s process. In a series of experiments, Haber started with known amounts of dinitrogen and dihydrogen maintained at high temperature and pressure and at regular intervals determined the amount of ammonia present. He was successful in determining also the concentration of unreacted dihydrogen and dinitrogen. Fig. 7.4 shows that after a certain time the composition of the mixture remains the same even though some of the reactants are still present. This constancy in composition indicates that the reaction has reached equilibrium. In order to understand the dynamic nature of the reaction, synthesis of ammonia is carried out with exactly the same starting conditions (of partial pressure and temperature) but using `color{red}(D_2)` (deuterium) in place of `color{red}(H_2)`. The reaction mixtures starting either with `color{red}(H_2)` or `color{red}(D_2)` reach equilibrium with the same composition, except that `color{red}(D_2)` and `color{red}(ND_3)` are present instead of `color{red}(H_2)` and `color{red}(NH_3)`. After equilibrium is attained, these two mixtures (`color{red}(H_2, N_2, NH_3)` and `color{red}(D_2, N_2, ND_3)`) are mixed together and left for a while. Later, when this mixture is analysed, it is found that the concentration of ammonia is just the same as before. However, when this mixture is analysed by a mass spectrometer, it is found that ammonia and all deuterium containing forms of ammonia (`color{red}(NH_3, NH_2D, NHD_2)` and `color{red}(ND_3)`) and dihydrogen and its deutrated forms (`color{red}(H_2, HD)` and `color{red}(D_2)`) are present. Thus one can conclude that scrambling of `color{red}(H)` and `color{red}(D)` atoms in the molecules must result from a continuation of the forward and reverse reactions in the mixture. If the reaction had simply stopped when they reached equilibrium, then there would have been no mixing of isotopes in this way.
Use of isotope (deuterium) in the formation of ammonia clearly indicates that chemical reactions reach a state of dynamic equilibrium in which the rates of forward and reverse reactions are equal and there is no net change in composition.
Equilibrium can be attained from both sides, whether we start reaction by taking, `color{red}(H_2(g))` and `color{red}(N_2(g))` and get `color{red}(NH_3(g))` or by taking
`color{red}(NH_3(g))` and decomposing it into `color{red}(N_2(g))` and `color{red}(H_2(g))`.
`color{red}(N_2 (g) +3H_2 (g) ⇌ 2NH_3 (g))`
`color{red}(2NH_3 (g) ⇌ N_2 (g) +3H_2 (g)) `
Similarly let us consider the reaction, `color{red}(H_2(g) + I_2(g) ⇌ 2HI(g))`. If we start with equal initial concentration of `color{red}(H_2)` and `color{red}(I_2)`, the reaction proceeds in the forward direction and the concentration of `color{red}(H_2)` and `color{red}(I_2)` decreases while that of `color{red}(HI)` increases, until all of these become constant at equilibrium (Fig. 7.5). We can also start with `color{red}(HI)` alone and make the reaction to proceed in the reverse direction; the concentration of `color{red}(HI)` will decrease and concentration of `color{red}(H_2)` and `color{red}(I_2)` will increase until they all become constant when equilibrium is reached (Fig.7.5). If total number of `color{red}(H)` and `color{red}(I)` atoms are same in a given volume, the same equilibrium mixture is obtained whether we start it from pure reactants or pure product.